Introduction
Raphanus raphanistrum subsp. sativus (radish) belong to a member of Brassicaceae family was a perennial plant and an edible root vegetable [1] (Fig. 1). According to the size, shape, and color of their thick roots of Raphanus raphanistrum subsp. sativus (radish), there were many different varieties available [1–4]. In general, the most usable and edible part of Raphanus raphanistrum subsp. sativus (radish) was the root, and also the leaves and stem had been utilized for flavoring or preservation of food [1,5]. The nutritional value of Raphanus raphanistrum subsp. sativus (radish) was composed of carbohydrates, high amounts of fiber, low amounts of fat, many essential minerals, vitamins, and so on [6]. Raphanus raphanistrum subsp. sativus (radish) was known to be very important as a medicinal property. Until now, Raphanus raphanistrum subsp. sativus (radish) was widely exploited around the world as traditional medicine for treatment such as ailments and disorders by various factors which included anemia, female (and/or male) infertility, gastrointestinal systems, respiratory, skin, urinary, and so on [1,7]. According to the report of Manivannan et al.Raphanus raphanistrum subsp. sativus (radish) had been reported to possess (1) antioxidant effects, (2) Hepatoprotective effects, (3) anticancer effects, (4) antidiabetic effects, and so on [8] (Fig. 2).

Especially, the leaves and roots of Raphanus raphanistrum subsp. sativus (radish) were used as antimicrobial substances [4,9]. It was generally know that the pharmacological characteristics of Raphanus raphanistrum subsp. sativus (radish) were caused by a wide range of 2nd metabolites. Secondary metabolites included alkaloids, antioxidant enzymes, carotenoids, coumarins, flavonoids (including anthocyanins), glucosinolates, phenolics, terpenes, and other compounds [10–13]. Furthermore, the usability of natural extracts has recently increased for improving human’s health [14].
In fact, the world’s most important issue was foodborne disease. Especially, various foodborne pathogenic bacteria could be easily spread to humans through milk (or dairy products) that could stimulate readily the outbreak of serious diseases [15]. Since milk (or dairy products) was recognized as the high nutritious food to improve the human’s health, milk (or dairy products) could easily become a habitat for foodborne pathogenic bacteria [15,16]. Therefore, attention would be focused on the safety of milk (or dairy products) [17,18]. Among various foodborne pathogenic bacteria, six different foodborne pathogenic bacteria such as Listeria monocytogenes ATCC 51776, Salmonela Enteritidis 110, Escherichia coli 23716, Cronobacter sakazakii KCTC 2949, Bacillus cereus ATCC 10876, and Staphylococcus aureus ATCC 6538 that were often found in milk (or dairy products) were selected and investigated.
Therefore, this study was aimed at investigating the antimicrobial action of Raphanus raphanistrum subsp. sativus (radish) on Listeria monocytogenes ATCC 51776, Salmonela Enteritidis 110, Escherichia coli 23716, Cronobacter sakazakii KCTC 2949, Bacillus cereus ATCC 10876, and Staphylococcus aureus ATCC 6538 so as to improve the quality of milk (or dairy products) and also to use it as food additives for preventing diabetes.
Materials and Methods
The powder of Raphanus raphanistrum subsp. sativus (radish) produced in Korea was purchased from Heungyildang Food (Korea). The dried powder of Raphanus raphanistrum subsp. sativus (radish) was macerated in 95% ethanol for at least two days (over 48 hours) with occasionally stirring at ambient temperature. And then, the soluble ingredients were concentrated by rotary evaporator at 50°C until dryness, and then the yield was obtained by ethanol extraction type. These stock solutions were filtrated through 0.2 mm Millipore and stored at —20°C before use.
Listeria monocytogenes ATCC 51776, Salmonela Enteritidis 110, Escherichia coli 23716, Cronobacter sakazakii KCTC 2949, Bacillus cereus ATCC 10876, and Staphylococcus aureus ATCC 6538 were obtained from KU Center for Food Safety, College of Veterinary, Konkuk University in Seoul, Korea. These bacteria were grown on nutrient agar (NA) (Oxoid, UK) overnight. Colonies were transferred into tubes containing cryopreservation fluid according to the instruction of the manufacturer (Original Microbiology Bead Storage System, STS, Technical Service Consultants Limited, UK). The beads were stored at —70°C until use.
The antibacterial action of Raphanus raphanistrum subsp. sativus (radish) were tested on Listeria monocytogenes ATCC 51776, Salmonela Enteritidis 110, Escherichia coli 23716, Cronobacter sakazakii KCTC 2949, Bacillus cereus ATCC 10876, and Staphylococcus aureus ATCC 6538 using by the spot-on-lawn assay with some modifications [19]. All test bacteria were cultured on Mueller-Hinton broth (MHB; Difco) and incubated at 37°C±0.5 for one day. The culture broth was diluted using MHB to 0.5 McFarland and spread onto Mueller-Hinton agar (MHA; Difco) using sterilized cotton swabs. A total of negative control (0 μL), 1× (10 μL), 2× (20 μL), and 3× (30 μL) of Raphanus raphanistrum subsp. sativus (radish) extract was directly dropped onto the surface of the MHA, respectively. The plates were incubated for one day at 37±0.5°C, and the inhibition zone was observed.
The inhibition of various concentration of Raphanus raphanistrum subsp. sativus (radish) ethanol extracts against Listeria monocytogenes ATCC 51776, Salmonela Enteritidis 110, Escherichia coli 23716, Cronobacter sakazakii KCTC 2949, Bacillus cereus ATCC 10876, and Staphylococcus aureus ATCC 6538 were analyzed by ANOVA (one-way analysis of variance), and all experiments were carried out independently in duplicate replications, and statistical significance was accepted at the p=0.05 level.
Results and Discussion
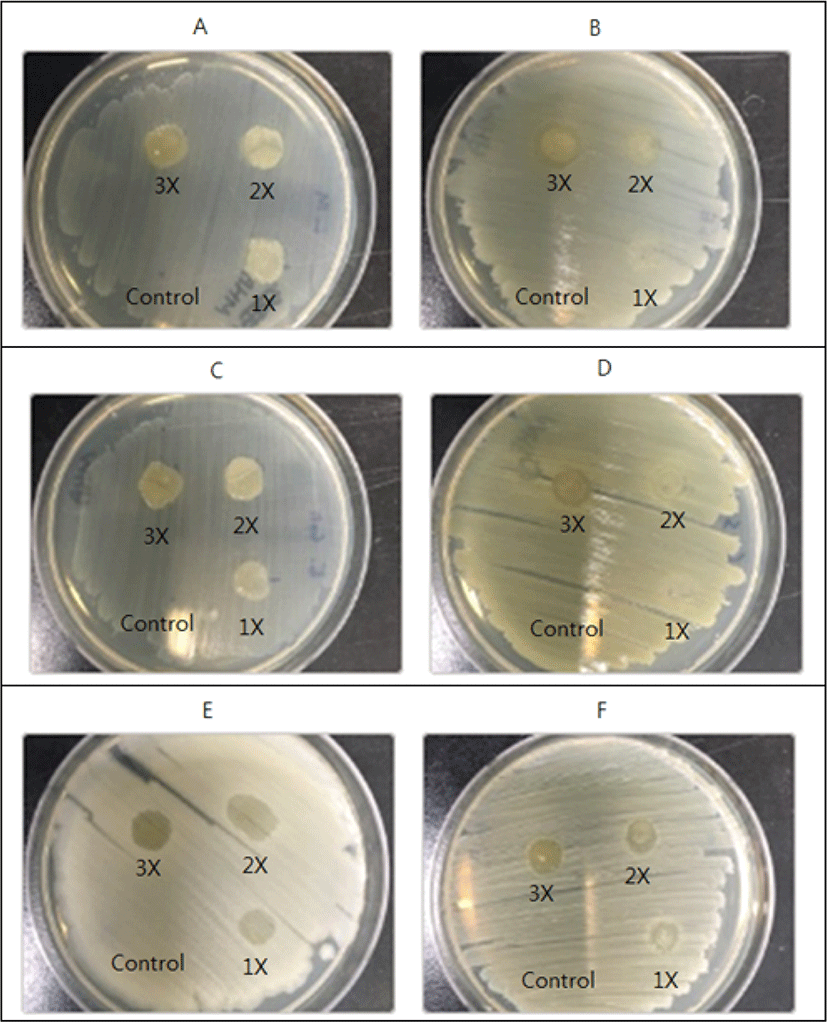
The ethanol extract of Raphanus raphanistrum subsp. sativus (radish) showed various levels (control, 1×, 2×, and 3×) of antimicrobial action when tested by the spot-onlawn assay (Fig. 3). These results obtained showed that ethanol extract of Raphanus raphanistrum subsp. sativus (radish) exhibited antimicrobial action against Salmonela Enteritidis 110 (partial inhibition), Cronobacter sakazakii KCTC 2949 (partial inhibition), Bacillus cereus ATCC 10876 (partial inhibition), and Staphylococcus aureus ATCC 6538 (total inhibition), and the inhibitory action of Raphanus raphanistrum subsp. sativus (radish) ethanol extracts was shown as a whole regardless of the increase in the concentration (Fig. 3). Although the final result of antimicrobial action of Raphanus raphanistrum subsp. sativus (radish) extract by ethanol against Listeria monocytogenes ATCC 51776 and Escherichia coli 23716 was negative, it did not seem to be effective at all (Fig. 3). In this study, it is likely that the higher concentration, the more effective it will be. Whereas the other two bacteria - Listeria monocytogenes ATCC 51776 and Escherichia coli 23716- did not show any inhibition by the ethanol extract of Raphanus raphanistrum subsp. sativus (radish) (Fig. 3).
Until now, it was being used in the treatment of various infection diseases using Raphanus raphanistrum subsp. sativus (radish) from the perspective of traditional medicine. These attempts have been a major stimulant to study of antimicrobial action of Raphanus raphanistrum subsp. sativus (radish) [1]. According to various previous researches, it had been shown that Raphanus raphanistrum subsp. sativus (radish) suppressed the growth of various bacteria, even those that were drug (or antibiotic)resistant [1]. Also, the root juice of Raphanus raphanistrum subsp. sativus (radish) could inhibit the growth of Bacillus subtilis, Enterococcus faecalis Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Pseudomonas pyocyaneus, Salmonella typhi, Staphylococcus aureus, and so on [20].
Using ethanol, ethylacetate, methanol, petroleum ether and water, crude extracts from the root-peel of Raphanus raphanistrum subsp. sativus (radish) black color showed the antimicrobial action against B. subtilis, Bordetella bronchiseptica, Enterobacter aerogenes, E. coli, K. pneumonia, Micrococcus luteus, P. aeruginosa, S. typhi, S. aureus, and so on [21]. Especially, the substances extracted from white and black peel taproots of Raphanus raphanistrum subsp. sativus (radish) using methanol could have suppress a wide variety of pathogenic bacteria associated with food which were Arthrobacter atrocyaneus, B. sphaericus, Corynebacterium ammoniagenes, Corynebacterium flavescens, Enterobacter hormaechei, Kocuria rosea, Neisseria subava, Pantoea agglomerans, Proteus vulgaris, Psychrobacter immobilis, Shigella dysenteriae, and so on [22]. Also, the substances extracted from the root, stem, and leaf of Raphanus raphanistrum subsp. sativus (radish) white color using chloroform, ethyl acetate and methanol exhibited significantly antimicrobial action against various foodborne and drug-resistant pathogenic bacteria such as B. subtilis, clinical isolates of E. cloacae, E. faecalis, E. aerogenes, E. coli, K. pneumonia, P. aeruginosa, S. epidermidis, S. typhimurium, S. aureus, and so on [23]. The substances extracted from seeds of Raphanus raphanistrum subsp. sativus (radish) white color using ethanol and methanol could strongly show the antimicrobial action against various pathogenic bacteria such as E. coli, K. pneumoniae, P. vulgaris, P. aeruginosa, S. aureus, S. sonnei, S. typhi, S. paratyphi, and so on [24]. Furthermore, the substances extracted from seeds of Raphanus raphanistrum subsp. sativus (radish) using methanol and water demonstrated the antimicrobial action against various plant pathogenic bacteria and fungi [25]. The results of this experiment also showed a similar trend to previous studies.
According to Shukla et al. [20], the root juice of Raphanus raphanistrum subsp. sativus (radish) showed the noticeable antimicrobial action against both gram-positive as Staphylococcus aureus and gram-negative as Enterococcus faecalis, Escherichia coli, Klebsiella pneumonia and Pseudomonas aeruginosa with the MIC range from 0.078 mg/mL to 0.625 mg/mL. On the other hand, the MIC range of ampicillin as standard antibiotic showed from 0.078 mg/mL to 0.312 mg/mL. The results of this experiment also showed a similar trend to previous studies. According to Jadoun et al. [1], the substances extracted from seeds of Raphanus raphanistrum subsp. sativus (radish) red color using ethanol demonstrated the antimicrobial action against 5 different pathogenic bacteria such as E. coli, K. pneumonia, S. pyogenes, S. typhimurium, and S. aureus with the inhibition zone from 9±2 mm to 20±2 mm. On the other hand, the inhibition zone of chloramphenicol (10 mg/mL) as standard antibiotic exhibited from 15±1 mm to 30±2 mm [1].
In fact, there were many studies for investigating the antimicrobial action of Raphanus raphanistrum subsp. sativus (radish). However, very little research had been done on the antimicrobial action of seeds of Raphanus raphanistrum subsp. sativus (radish) red color [1]. On the contrary, according to other previous studies, the substances extracted from seeds of Raphanus raphanistrum subsp. sativus (radish) using mixture of dichloromethane and methanol demonstrated the lack of antimicrobial action against 14 different bacterial and fungal strains [26]. But, Jadoun et al. addressed that crude substances extracted from seed of Raphanus raphanistrum subsp. sativus (radish) was considerably effective against all pathogenic bacteria tested [1]. It was thought that the different findings between the two studies may be due to reason of (1) the extraction methods and (or) the solvents used [22,24,27].
Especially, according to report of Jamuna et al. [27], the cold extraction of fresh root of Raphanus raphanistrum subsp. sativus (radish) was significantly better than dried cold extraction or soxhlet in antioxidant activities tested. Also, according to study of Ahmad et al. [24], the antimicrobial action against 8 different pathogenic bacteria including gram-positive and gram-negative was better in extracts of seed of Raphanus raphanistrum subsp. sativus (radish) white color using ethanol and methanol than in acetate, benzene, chloroform, ethyl acetate, and water. Furthermore, Janjua et al. reported there were differences in antimicrobial action depending on the solvent used such as ethanol, ethyl acetate or others in peels of Raphanus raphanistrum subsp. sativus (radish) black color [21]. One thing to be careful about was that the antimicrobial action of the substances extracted from various solvents varied widely, especially depending on the species of bacteria and on genetic variation [21].
Therefore, the potential antimicrobial action by the unknown plant-derived substance became increasingly interested so as to prevent the outbreak of various drug-resistant pathogenic bacteria [28]. Especially, the substances extracted from various plants should be widely examined for its possibility for natural biopreservatives such as antimicrobial action, since many consumers urgently needed for natural preservatives for substituting artificially manufactured chemicals [1,6]. Also, research on various substances extracted from various Raphanus raphanistrum subsp. sativus (radish) produced in each country would have to be carried out.
In conclusion, this study demonstrated the potential of Raphanus raphanistrum subsp. sativus (radish) to inhibit the growth of Salmonela Enteritidis 110, Cronobacter sakazakii KCTC 2949, Bacillus cereus ATCC 10876, and Staphylococcus aureus ATCC 6538 as antimicrobial action, except for Listeria monocytogenes ATCC 51776 and Escherichia coli 23716. Hence, this study indicated that Raphanus raphanistrum subsp. sativus (radish) could be applicable in natural biopreservatives such as antimicrobial action for improving the food safety, and also in functional food material of the food industry.
