Introduction
Koumiss is a traditional, fermented milk drink originating from the nomadic tribes of Central Asia (China, Kazakhstan, Krgyzstan, etc) and Russia (Kucukcetin et al., 2002; Hao et al., 2010; Mu et al., 2012). Although a product with a long history, research on Koumiss has been limited (Lee et al., 2011; Zhao et al., 2011). The product is unique among dairy foods in that it is made using mare’s milk (Sari et al., 2014). Starter culture used include a variety of lactic acid bacteria and yeasts (Mu et al., 2012). Similar to Kefir, both lactic acid bacteria and alcohol fermentation occur in Koumiss (Sari et al., 2014). However, unlike Kefir, there is no grain structure of the Koumiss starter (Lee et al., 2011; Sari et al., 2014). Traditionally, the natural starter culture is the previously fermented Koumiss (Sabancı et al., 2016). Three types of Koumiss as strong (pH 3.3-3.6), moderate (pH 3.9-4.5), and light (pH 4.5-5.0) based on the lactic acid content were reported (Danova et al., 2005). Different acid content was attributed to different lactic acid bacteria cultures used in the production of Koumiss. Koumiss consumption is limited to a specific geographic area and has not been globally commercialized (Wang et al., 2008; Lee et al., 2011; Zhao et al., 2014). In areas where it is widely consumed, Koumiss has been traditionally considered a health-prompting product that improves metabolism and protects the nervous system and kidneys. Di Cagno et al. (2004) cited a reference suggesting use of mare’s milk for allergic children. Twenty-one stains of Lactobacillus from Koumiss were isolated and 16 strains produced ACE (angiotension I-converting enzyme) inhibitory activity and 2 strains produced γ-aminobutyric acid (GABA). ACE activity plays an important role in the regulation of blood pressure. ACE inhibitors and GABA have antihypertensive effect (Di Cagno et al., 2004).
Among various medicinal plants currently available, Cichorium intybus L. (chicory) is a vegetable largely consumed in the world as such for its tonic effects on the liver and the digestive tract (Kang et al., 2016; Jeong et al., 2016). For example, Cichorium intybus L. (chicory) has been stated as possessing tonic properties in Indian traditional medicine (Street et al., 2013). Previous studies have also shown that Cichorium intybus L. (chicory) preparations exert a potent anti-hepatotoxic activity (Zafar and Mujahid Ali, 1998). Alcoholic extracts of Cichorium intybus L. (chicory) have been used to treat pyorrhea or gingival inflammation (Pushparaj et al., 2007). It has been reported to display a quinidine-like action on the isolated heart (Pushparaj et al., 2007). In addition, it appears that Cichorium intybus L. (chicory) extracts are widely used to treat hyperglycemia in Europe and North America (Pushparaj et al., 2007). Furthermore, an hypoglycemic effect of Cichorium intybus L. (chicory) has been reported in streptozotocin-like diabetic rats (Kamel et al., 2011). Also chicoric acid root extract is able to promote both insulinotropic and insulin sensitizing effects (Carazzone et al., 2013).
Hence, the purpose of this study was to produce the functional Koumiss added with Cichorium intybus L. (chicory) for upgrading sensory evaluation as new concept having various health benefits of Koumiss. So, we analyzed pH, TA and sensory evaluation of the functional Koumiss added with Cichorium intybus L. (chicory) produced in this experiment.
Materials and Methods
Cichorium intybus L. (chicory)’s roots were supplied by Center for One Health, Konkuk University in Seoul, Korea and then were cleaning, cutting into slices, and drying, respectively. And the dried roots were macerated in 95% ethanol for 48 hours with occasionally stirring at ambient temperature (ca. 25℃) and hence the soluble ingredients were concentrated by rotary evaporator (Rotavapor® R-100, BUCHI Corp., USA) at 50℃ until dryness. The yield obtained was obtained for ethanol extraction type. These stock solutions were filtrated through 0.2 mm millipore and stored at -20℃ before use.
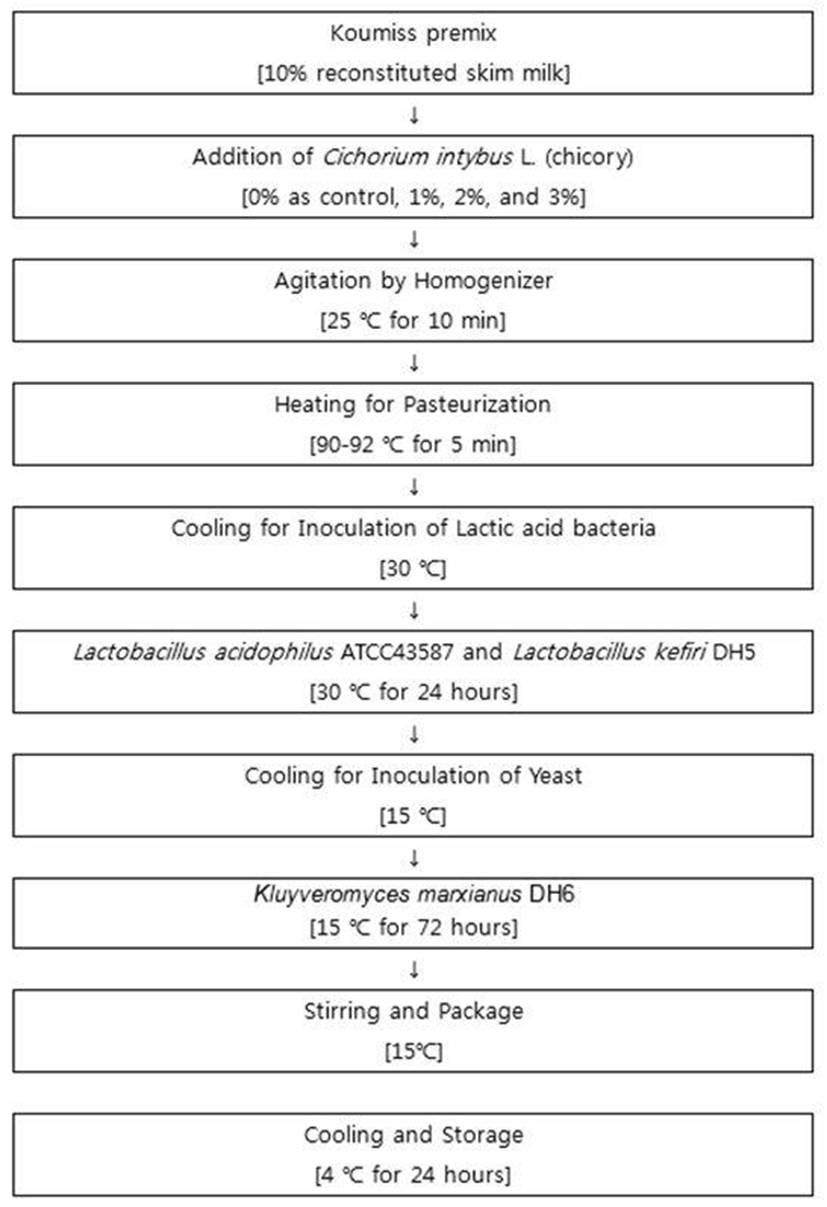
Crude materials extracted from Cichorium intybus L. (chicory) was added to premix of Koumiss at concentrations of 0% (control), 1, 2, 3% and then homogenized (T 25 digital ULTRA-TURRAX®, IKA-Labortechnik, Staufen, Germany). The lactic acid bacteria (Lactobacillus acidophilus ATCC 43587, Lactobacillus kefiri DH5) and yeast (Kluyveromyces marxianus DH6) were inoculated and fermented, and then the functional Koumiss was stored at 4℃ for 24h. The functional Koumiss was made by modification of Lee et al.(2011) (Fig. 1).
The titratable acid (TA) was determined by titration with 0.1 N NaOH, and the pH of the homogenized yoghurt was determined using a digital pH meter (Orion Star A211, USA) according to method of Jeong et al.(2016).
The sensory evaluation was carried out by 10 trained evaluators between 20 and 40 years of age. The samples were coded with three digit numbers and randomly served at 7 to 10℃ in plastic cups (10 mL). All evaluators completed a test assessment form to compare the five sensory attributes (appearance, flavor, taste, and overall acceptability) by using a five-point hedonic scale (1, extremely poor; 2, poor; 3, fair; 4, good; 5, excellent). More details please refer to Table 2.
Two separate experiments with triplicate assays were performed. Data were expressed as means. Statistical analysis was performed using one-way analysis of variance (ANOVA; SPSS 19.0, USA) followed by Duncan’s post hoc test for mean comparison. Statistical significance was established as p<0.05.
Results and Discussion
The TA was increased to around 0.85 to 0.88% after the fermentation of Koumiss premix (Data not shown).The TA contents of the functional Koumiss added with Cichorium intybus L. (chicory) (1 to 3%) showed similar to that of conventional Koumiss as control (Data not shown). On the other hand, the pH was decreased to around 4.34 to 4.35 after the fermentation of Koumiss premix (Data not shown). The pH value of the functional Koumiss added with Cichorium intybus L. (chicory) (1 to 3%) showed similar to that of conventional Koumiss as control (Data not shown). The pH and TA contents of the functional Koumiss added with Cichorium intybus L. (chicory) showed similar to those of commercial Koumiss reported by previous studies (Table 1) (Robinson et al., 2002; Danova et al., 2005; Lee et al., 2011; Carrick, 2012; Zhang and Zhang, 2012).
| Type of Koumiss | pH | Titratable acidity | Alcohol contents |
|---|---|---|---|
| Strong | 3.3~3.6 | 0.91~1.08 | 1.8~2.5 |
| Moderate | 3.9~4.5 | 0.73~0.90 | 1.1~1.8 |
| Light (weak) | 4.5~5.0 | 0.54~0.72 | 0.7~1.0 |
Resources: Robinsone et al., 2002; Danova et al., 2005
In this study, there's no significant difference among these 3 groups including control group in pH and TA, and also pH is high depending on the increase of TA. Also the results of this experiment showed a similar pattern with the results of previous other experiments.
The sensory evaluation of the yoghurt was evaluated by 10 trained evaluators of ages 20 to 40 years, and the results are summarized in Table 2.
The functional Koumiss was prepared with Cichorium intybus L. (chicory) at concentrations of 0%, 1%, 2%, and 3%, respectively. The taste scores for the functional Koumiss with Cichorium intybus L. (chicory) (1 to 3%) ranged from 2.8 points to 2.5 point, which were lower than those for conventional Koumiss without addition of Cichorium intybus L. (chicory) (0% as control) (2.9 points). The flavor score of the functional Koumiss with Cichorium intybus L. (chicory) (1 to 3%) ranged from 2.5 points to 2.3 point, whereas that of the conventional Koumiss without addition of Cichorium intybus L. (chicory) (0% as control) showed 2.9 points. The color value of the functional Koumiss with Cichorium intybus L. (chicory) (1 to 3%) ranged from 4.3 points to 3.9 point, which was comparable to that of conventional Koumiss without addition of Cichorium intybus L. (chicory) (0% as control) showed 4.7 points. The texture of the functional Koumiss with Cichorium intybus L. (chicory) (1 to 3%) ranged from 3.4 points to 3.3 point, which was comparable to that of conventional Koumiss without addition of Cichorium intybus L. (chicory) (0% as control) showed 3.6 points. And the overall acceptability of the functional Koumiss with Cichorium intybus L. (chicory) (1 to 3%) ranged from 3.3 points to 3.0 point, which was comparable to that of conventional Koumiss without addition of Cichorium intybus L. (chicory) (0% as control) showed 3.5 points. Namely, the sensory evaluation decreased with increasing amounts of added Cichorium intybus L. (chicory). Among the experimental group, high scores were received by Cichorium intybus L. (chicory) - containing Koumiss with 1% compared with the control group. Summarizing the results, the taste, flavor, color, texture, and overall acceptability decreased in proportion to the added amount of Cichorium intybus L. (chicory) (Table 2).
When compared to similar study, according to Lee et al. (2011), Korean-type Koumiss was produced by the fermentation of mixed cultures (1:1 ration of Kuyveromyces and lactic acid bacteria consisted of Lactobacillus bulgaricus & Streptococcus thermophiles) were inoculated into 10% skimmed milk with addition of whey powder (control, 2%, 4%, 6%, and 8%). As the dosage of whey powder increased, fat, protein, lactose, titratable acidity, the number of lactic acid bacteria and yeast, alcohol content showed a tendency to gradually increase (Lee et al., 2011). Also, the scores increased as whey powder content increased in sensory evaluations, and there were no great differences among the samples in the case of appearance (Lee et al., 2011). Koumiss produced by fermented mare’s milk generally contained Lactobacillus bulgaricus and Saccaromyces lactis and Torula spp., and also has been used to treat tuberculosis, gastric and intestinal diseases, avitaminosis, anemia and disease of the liver and kindey (Dilanyan, 1959). According to report of Sari et al.(2014), the weights of the mice in Koumiss were increased comparted to controls, and Koumiss increased PPARα and PPAR-β/δ expressions. Peroxisome proliferator-activated receptors (PRARs) was consisted of three subtypes of PRARα, PRARβ/δ, and PRARγ, and furthermore is known to play a significant role in cell differentiation, regulation of lipid metabolism, energy balance, inflammation and arteosclerosis. Also Hao et al. (2010) firstly revealed that L. bucheri, L. jensenii and L. kitasatonis were identified using by denaturing gradient gel electrophoresis and species-specific polymerase chain reaction in Koumiss, because thsse 3 strains were never previously isolated by culture-depending methods,.
Root of Cichorium intybus L. (chicory) have been reported to exert antidiabetic benefits in Eurasis people (Street et al., 2013). For example, a natural chicoric acid extract (NCRAE) from Cichorium intybus L. (chicory)’s root has been shown to increase insulin secretion by pancreatic β- cells and glucose uptake by muscle cells. In 1958, Scarpati and Oriente(1958) isolated and identified a phenolic compound from the leaves of chicory (Cichorium intybus L.) plants, and that was a tartaric acid ester of two caffeic acids (a hydroxycinnamic acid), and then it was named as chicoric acid (Fig. 2).
Since that first discovery, chicoric acid has since been charted in many plant families, including those of seagrass, horsetail, fern, lettuce, and basil(Lee and Scagel, 2013). In general, chicoric acid helped a plant protect itself from insects and infection from viruses, bacteria, fungi, and nematodes and that it aided in wound healing in plants after mechanical damage(Lee and Scagel, 2013). Also, Azay-Milhau et al.(2013) reported that a natural chicoric acid extract (NCRAE) from Cichorium intybus L. (chicory)’s root presents an antihyperglycemic effect essentially due to a peripheral effect on muscle glucose uptake. And Nishimura et al.(2015) reported that Cichorium intybus L. (chicory)’s root extract delay or prevent the early onset of diabetes mellitus and improve bowel movement. Cichorium intybus L. (chicory) has inulin which has various pharmacological effects. For example, in a clinical study of women with type 2 diabetes, inulin supplementation for 2 months improved fasting plasma glucose, insulin, and hemoglobin A1c levels, and decreased malondialdehyde levels compared with maltodextrin supplementation (Pourghassem Gargari et al., 2013). Another study reported that ingestion of inulin for 2 weeks was well tolerated by adult participants and led to a significant improvement of bowel movements with a minimum effect on fecal microflora (García-Peris et al., 2012).
In summary, the functional Koumiss added with 0%, 1%, 2%, and 3% of Cichorium intybus L. (chicory) showed the increase of TA and decrease of pH, simultaneously. Also the functional Koumiss containing 1% concentration of Cichorium intybus L. (chicory) received higher scores for taste, flavor, texture and overall acceptability in the sensory evaluation. Therefore, it needs further studies so as to produce multi-purpose functional Koumiss through upgraded availability of Cichorium intybus L. (chicory) for prompting health.
Disclaimer: The views expressed herein do not necessarily reflect those of the US Food and Drug Administration or the US Department of Health and Human Services







